При разложении карбоната кальция образовалось 50 грамм оксида кальция и выделился углекислый газ. найти v и m углекислого газа. caco3=cao+co2
Другие вопросы по теме Химия
Популярные вопросы
- (каз.яз.) я люблю ходить в кино и смотреть различные фильмы. мне побольшому...
1 - Сделать фонетический разбор шлепать...
3 - Требовалось написать программу, которая вводит с клавиатуры последовательность...
1 - Найди длины отрезков ab ac ad если радиус каждой окружности 1 см 8 мм...
1 - Распределите слова в два столбика take или get пример: take: a test get:...
1 - Каким образом животные при к обитанию водной среды надо!...
3 - Сумма трёх чисел 1006542 . одно слагаемое - наименьшее пятизначное , второе...
1 - Сумма третьего и шестого членов арифметической прогрессии равна 3. второй...
3 - Какой документ отражает основные направления экологической политики россии?...
3 - Напиши пьесу комедию как в ! надо...
2
CaCO3=CaO+CO2
n CaO=50/56=0.893 моль
n CO2=0.893 моль
V CO2=0.893*22.4=20 л
m CO2=20*1.97=39.54г
50 x1\x2
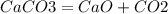
56 22.4\44
x1(V) = 22.4*50/56 = 20 л
x2(m) = 44*50/56 = 39.2 гр