25 решите . к 50 мл 96% раствора серной кислоты (р=1,84 г/мл) прибавили 50 мл воды. определите процентное содержание растворенного вещества в полученном растворе. б) определите молярную концентрацию 72% раствора азотной кислоты (р = 1,43 г/мл).
Ответы
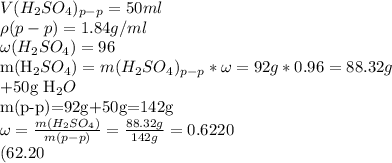 ) +50ml H_{2}O " alt="m(H_{2}SO_{4})_{p-p}=V(H_{2}SO_{4})_{p-p}*\rho=50ml*1.84g/ml=92g m(H_{2}SO_{4})=m(H_{2}SO_{4})_{p-p}*\omega=92g*0.96=88.32g +50g H_{2}O m(p-p)=92g+50g=142g \omega= \frac{m(H_{2}SO_{4})}{m(p-p)} = \frac{88.32g}{142g} =0.6220 (62.20 %)" />) +50ml H_{2}O " />
) +50ml H_{2}O " alt="m(H_{2}SO_{4})_{p-p}=V(H_{2}SO_{4})_{p-p}*\rho=50ml*1.84g/ml=92g m(H_{2}SO_{4})=m(H_{2}SO_{4})_{p-p}*\omega=92g*0.96=88.32g +50g H_{2}O m(p-p)=92g+50g=142g \omega= \frac{m(H_{2}SO_{4})}{m(p-p)} = \frac{88.32g}{142g} =0.6220 (62.20 %)" />) +50ml H_{2}O " />
Другие вопросы по теме Химия
Популярные вопросы
- Шолохов они сражались за родину краткое содержание. 2-3 предложения...
2 - Написать сочинение на тему, каникулы в югре...
2 - Как ты считаешь, ущемлены ли права ребёнка (птенца пингвина)....
3 - При каком значении с система уравнений 6х +су =2 12х+5у=5 не имеет...
2 - Тебе конечно знакома картина шишкина утро в сосновом лесу . нравится...
2 - Водном из ниже слов допущена ошибка в образовании формы слова....
3 - Напишите пересказ по событиям валерий медведев баранкин, будь...
2 - Бойындағы ең жақсы қасиеттер бізге алдымен анадан тарайды. сөйлемге...
1 - Сумма 2 чисел равно 48, найдите числа, если одно число равно 40%...
3 - На гладком столе находится тело массой 2 кг. другое тело массой...
3