Вычислите массовые доли элементов в фосфорной кислоте H3PO4
Другие вопросы по теме Химия
Популярные вопросы
- Какие завезенные колонистами животные нарушили равновесие в природных...
2 - «Шестое чувство» (ответьте на вопросы) за это...
1 - Золушка на балу потеряла хрустальную туфельку Определите плотность...
3 - 4 Сандардын ретін анықта.Қай бала дұрыс тұрған жоқ? өз сандар тізбегіңді...
2 - Задания 2 уровень 1)xy+2y – 2х - 4 3 уровень 1) x+xy-xy-у 1 уровень...
1 - При якому натуральному значенни х обидва дроби 4х+5/33 и 7/х е неправильними...
2 - 7. Визначте, які наведені ознаки властиві антициклону: а) атмосферний...
2 - Завязка, развитие сюжета кульминация, развязка (Снежная Королева)...
3 - Сергей Лукьяненко «Девочка с китайскими зажигалками» Вопрос: «Что...
3 - Скобка открывается 1целая 7/10+4/5):1 целая7/8...
3
W(H)=3.06%, W(P)=31.63%, W(O)=65.31%
Объяснение:
W(H3PO4)=W(H)+W(P)+W(O)
W(H3PO4)=1*3+1*31+4*16
W(H3PO4)=98
W(H)=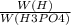 =
=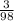 *100%=3.06%
*100%=3.06%
W(P)=\frac{W(P)}{W(H3PO4)} =\frac{31}{98}**100%=31.63%
W(O)=100%-3.06%-31.63%=65.31%