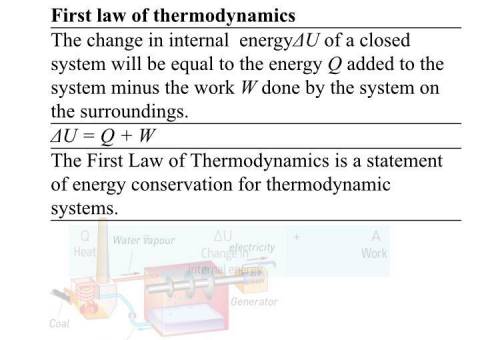
The change in internal energyΔU of a closed system will be equal to the energy Q added to the system minus the work W done by the system on the surroundings.
ΔU = Q + W
The First Law of Thermodynamics is a statement of energy conservation for thermodynamic systems.
Ответы
Показать ответы (3)
Другие вопросы по теме Физика
Популярные вопросы
- Выражение 5/8a*4/15d, 6 3/4x*1 11/45 y, 13/24d*32c, 18 1/3a*1...
2 - Сообщение временное правительство и советы и о самом главном...
3 - Вставляя пропущенные буквы, спишите словосочетания сначала с...
2 - Сочинение на тему мороз красный нос где можно найти?...
3 - Составить описание картины а,пластова сенокос...
1 - Опишите одного из мальчиков из рассказа бежин луг. объясните...
1 - Надо сделать 7 вопросов про макал мадениет на казахском...
2 - Напишите контрольную работу по на тему площади где есть: 3.вопроса...
3 - Выбираете функции: графики которых параллельны: графики которых...
1 - Евгелена зелёная имеет знаки животных и...
3